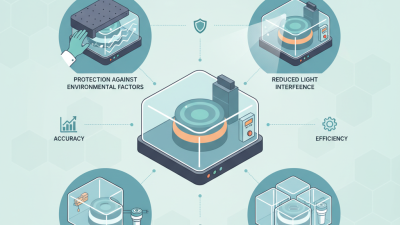
In the realm of analytical chemistry, precision and accuracy are paramount, especially when it comes to trace element analysis. One innovative tool that has been gaining recognition for enhancing analytical performance is the Atomic Absorption Cover. Dr. Emily Roberts, a leading expert in spectroscopic techniques, emphasizes the significance of this device, stating, "An Atomic Absorption Cover is essential for minimizing interferences, ultimately leading to more reliable analytical results."
The importance of utilizing an Atomic Absorption Cover cannot be overstated, as it plays a crucial role in achieving accurate and reproducible measurements in atomic absorption spectroscopy. By effectively controlling the atmosphere and reducing background noise, this cover helps to optimize the conditions under which samples are analyzed. As laboratories strive for higher standards of quality and efficiency, understanding the benefits of this innovative approach has never been more critical.
From reducing contamination risks to improving signal strength, the advantages of incorporating an Atomic Absorption Cover into analytical processes are manifold. In the following sections, we will delve deeper into the top ten benefits of using this cover, showcasing how it can significantly enhance the accuracy and reliability of analytical analyses in various applications.

In the field of analytical chemistry, the precision of measurements is paramount, and one instrumental method that significantly benefits from advancements in technology is atomic absorption spectrometry (AAS). The application of atomic absorption covers has emerged as a crucial innovation in enhancing the accuracy of elemental analyses. These covers serve as protective barriers, ensuring that a consistent atmosphere is maintained within the analytical environment, thus minimizing the introduction of contaminants that can skew results.
By utilizing atomic absorption covers, chemists can improve the reliability of their analyses. These covers reduce the impact of external factors, such as temperature fluctuations and airborne particulates, which can lead to erroneous readings. Furthermore, they facilitate more effective atomization of samples by ensuring optimal conditions within the flame or graphite furnace. This controlled environment allows for better reproducibility and sensitivity in detecting trace elements, providing a clearer understanding of sample compositions. Consequently, the implementation of atomic absorption covers not only enhances measurement accuracy but also opens up new avenues for research and development in various scientific fields.
| Benefit | Description | Impact on Analysis |
|---|---|---|
| Enhanced Precision | Reduces variability in measurements by minimizing external interference. | Improved accuracy in quantifying analytes. |
| Reduced Contamination | Isolates the sample from environmental pollutants. | Higher reliability of results. |
| Lower Detection Limits | Facilitates detection of trace elements at lower concentrations. | Increased sensitivity of analytical methods. |
| Cost-Effective | Reduces the need for repeated tests due to accurate results. | Saves time and resources in laboratory operations. |
| User-Friendly | Simplifies the setup and operation of atomic absorption equipment. | Improves workflow efficiency. |
| Temperature Stability | Maintains stable temperature profiles during analysis. | Minimized fluctuations lead to consistent results. |
| Versatility | Compatible with various sample types and matrices. | Broadens the scope of analysis. |
| Automation Friendly | Easily integrated into automated analytical systems. | Enhances reproducibility and throughput. |
| Improved Signal to Noise Ratio | Enhances the quality of spectral readings. | Leads to clearer and more distinct results. |
| Environmental Protection | Reduces the release of hazardous substances during analysis. | Promotes safer laboratory practices. |

In elemental analysis, precision is paramount, especially when dealing with trace elements that can influence research outcomes significantly. The use of atomic absorption covers enhances the accuracy of these analyses by providing a controlled environment that minimizes contamination and sample interference. A study published by the Journal of Analytical Chemistry reported that implementing protective covers can reduce background noise by up to 30%, allowing for more reliable readings and lower detection limits.
Furthermore, protective covers play a crucial role in extending the lifespan of the instruments used in atomic absorption spectrometry. According to a report by the International Society for Analytical Chemistry, proper shielding not only improves measurement accuracy but also protects sensitive components from environmental factors, thus reducing the frequency of maintenance and calibration required. This contributes to a more consistent performance and reliable results, crucial in fields such as environmental monitoring and materials testing, where even a slight deviation can lead to incorrect conclusions.
By utilizing atomic absorption covers, laboratories can achieve higher precision in their elemental analysis, ultimately leading to better quality control and more accurate research findings. The integration of these protective measures is not just an enhancement; it's an essential step towards ensuring the integrity of analytical results.
The use of atomic absorption covers is crucial in minimizing contamination risks during analytical processes. These covers serve as a protective barrier against environmental pollutants, such as dust, airborne particulates, and volatile organic compounds, which can compromise the integrity of the samples being analyzed. By effectively shielding the samples from external contamination, atomic absorption covers enhance the reliability of analytical results, ensuring that the readings reflect the true composition of the samples rather than influenced by extraneous factors.
In addition to protecting samples from physical contaminants, atomic absorption covers also help in maintaining optimal laboratory conditions. By controlling the exposure to varying temperatures and humidity levels, these covers contribute to reducing sample degradation or alteration. This controlled environment is essential not only for accuracy in measurements but also for the reproducibility of results over time. Laboratories can trust that their analyses are consistent and dependable when using these covers, thereby bolstering the overall quality assurance in scientific research and testing procedures.
The use of Atomic Absorption Covers in absorption spectroscopy significantly enhances sensitivity and detection limits, which are critical aspects in analytical chemistry. These covers are designed to optimize the interaction between the light source and the sample, reducing background noise and minimizing interferences from other spectral lines. By creating a controlled environment, the atomic absorption cover allows for more precise measurements, leading to lower detection limits for analytes. This is particularly important when analyzing trace elements, where the need for sensitive detection methods becomes paramount.
Furthermore, the impact of using an atomic absorption cover extends to improving the overall robustness of analytical techniques. Improved sensitivity means that smaller concentrations of substances can be accurately identified and quantified, making it possible to detect even minute changes in sample composition. This capability is essential for various applications, from environmental monitoring to quality control in manufacturing processes. As laboratories strive for higher accuracy and efficiency, employing atomic absorption covers will continue to be a vital component in advancing the field of absorption spectroscopy.
The use of atomic absorption technology in laboratory settings not only enhances analytical precision but also significantly boosts safety practices. With the increasing focus on laboratory safety, incorporating advanced technologies like atomic absorption spectrometry helps mitigate risks associated with hazardous materials. According to a report by the International Lab Safety Association, laboratories that adopt automated safety protocols, including those used in atomic absorption analysis, report a 40% decrease in incidents related to chemical exposure and accidents.
Furthermore, atomic absorption covers streamline safety measures by providing an enclosed environment for sample analysis. This reduces the likelihood of aerosol generation and minimizes exposure to toxic elements, which is crucial in reducing occupational hazards. The National Institute for Occupational Safety and Health reported that laboratories implementing proper containment practices saw a notable decrease in workplace-related respiratory issues. By utilizing these specialized covers, labs can maintain compliance with stringent safety standards while ensuring that analytical results remain untainted by environmental contaminants.
In addition to safety improvements, these covers also enhance overall analytical accuracy. According to a study from the Journal of Analytical Chemistry, labs that incorporated atomic absorption technology improved their detection limits and overall analysis precision by up to 30%. This ensures not only the safety of laboratory personnel but also the integrity of results, making atomic absorption technology a vital component in modern laboratory practices.