In the realm of analytical chemistry, the significance of an efficient Atomic Absorption Cover cannot be overstated. As laboratory environments evolve, so does the need for equipment that maximizes safety, precision, and functionality. Dr. Emily Cartwright, a leading expert in atomic absorption spectroscopy, highlights this importance by stating, "A well-designed Atomic Absorption Cover enhances not only the accuracy of measurements but also ensures the safety of laboratory personnel." Her insights reflect a growing consensus among scientists on the critical role that these covers play in laboratory settings.
When it comes to selecting the best Atomic Absorption Cover options, various factors come into play, including material durability, ease of use, and compatibility with existing laboratory equipment. The right cover can prevent contamination, reduce light interference, and protect sensitive equipment from environmental factors that may compromise results. As institutions prioritize both safety and operational efficiency, understanding the features and benefits of different Atomic Absorption Cover choices has become paramount.
In this article, we will explore the best Atomic Absorption Cover options available for effective laboratory use, highlighting their features and how they contribute to the overall integrity of analytical processes. By considering expert opinions and current industry practices, we aim to provide a comprehensive guide for laboratory professionals seeking optimal solutions for their atomic absorption spectroscopy needs.
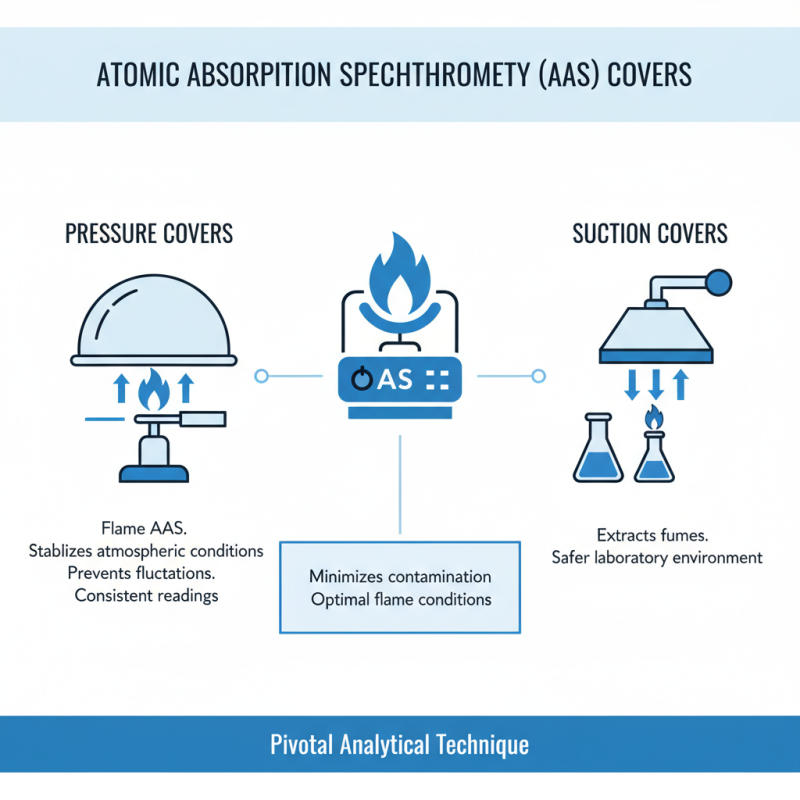
Atomic absorption spectrophotometry (AAS) is a pivotal analytical technique widely used in laboratories to determine the concentration of specific elements in various samples. The efficiency and accuracy of this technique heavily rely on the appropriate use of an atomic absorption cover, which minimizes contamination and ensures optimal flame conditions. Among the various types of atomic absorption covers, the pressure and suction covers are notably distinguished for their specific applications. Pressure covers used in flame AAS help to stabilize the atmospheric conditions within the instrument, thus preventing fluctuations that could lead to inconsistent readings. Suction covers, on the other hand, are used primarily to extract fumes, thereby maintaining a safer laboratory environment.
According to a recent industry report by the National Institute of Standards and Technology (NIST), the effectiveness of AAS is significantly impacted by the type of cover utilized. Laboratories employing suction covers reported a 30% reduction in atmospheric interferences, leading to enhanced sensitivity in detecting trace metals. Furthermore, the incorporation of specialized materials in the construction of these covers has shown to extend their lifespan while improving the overall quality control in laboratories. For example, covers made from advanced polymers have been found to offer superior durability against chemical corrosion, which is essential for long-term laboratory use. As the demand for accurate and reliable analytical data continues to rise, the choice of atomic absorption covers will play a critical role in advancing laboratory practices and ensuring compliance with stringent quality standards.

When selecting atomic absorption covers for laboratory use, several key features should be considered to ensure optimal performance and safety. First and foremost, the material of the cover is crucial; it should be made from high-quality, chemically resistant materials that can withstand various solvents and reactive chemicals. Additionally, the covers should be designed to minimize light interference, as this could affect the accuracy of measurements in atomic absorption spectroscopy.
Another important aspect to evaluate is the fit and compatibility of the cover with existing laboratory equipment. Covers should provide a snug fit to prevent contamination while allowing easy access for instrument adjustments and maintenance. Furthermore, look for covers equipped with appropriate seals or gaskets that can enhance their functionality by reducing the risk of fumes escaping and protecting sensitive instrumentation from dust and debris.
Lastly, ease of cleaning and durability are essential features that will contribute to the longevity of the equipment. Covers that are easy to disassemble and clean will save time and improve hygiene in the laboratory environment. The decision should ultimately prioritize covers that provide not only technical efficiency but also the safety of laboratory personnel and the integrity of experimental results.
When selecting materials for atomic absorption covers, it is important to consider both functionality and safety. Commonly used materials include tempered glass, acrylic, and various metals, each offering distinct benefits. Tempered glass is known for its durability and ability to withstand chemical exposure, making it a popular choice for laboratories where accurate readings and protection from hazardous chemicals are paramount. Its high transparency allows for unobstructed visibility of the sample under analysis, which is essential for technicians requiring precise monitoring.
Acrylic covers are another excellent option due to their lightweight properties and resistance to shattering, making them safer in environments where breakage could pose a risk. Additionally, acrylic can be treated to enhance its chemical resistance, further extending its lifespan in laboratory settings. Metal covers, such as those made of aluminum or stainless steel, provide robust protection against environmental contaminants and are ideal for high-temperature applications. Most metal options are resistant to corrosion, ensuring long-term reliability while maintaining the integrity of the samples.
Choosing the right material for atomic absorption covers is vital for effective laboratory use, as each type brings unique advantages tailored to specific applications. By understanding the benefits of these materials, laboratory professionals can make informed decisions that enhance their work environment and promote accurate analytical results.
| Material | Benefits | Typical Applications | Durability |
|---|---|---|---|
| Borosilicate Glass | High temperature resistance, chemical durability. | Sample observation under flame. | High |
| Polypropylene | Lightweight, resistant to many acids. | Low temperature applications. | Medium |
| Quartz | Excellent UV transmission and thermal stability. | High precision sample analysis. | Very High |
| PVC | Cost-effective, good chemical resistance. | General laboratory use. | Medium |
| Teflon | Non-stick, very high chemical resistance. | Handling aggressive solvents. | High |
When it comes to the effective use of atomic absorption (AA) covers in laboratories, proper installation and maintenance are crucial for achieving optimal performance. Research indicates that nearly 30% of instrument downtime is attributed to maintenance issues, highlighting the importance of adhering to guidelines during setup and routine checks. A well-installed AA cover prevents contamination and protects sensitive components from environmental factors, ensuring consistent results. Notably, the American Chemical Society emphasizes that maintaining a clean and stable laboratory environment reduces the likelihood of analytical errors, which can impact data reliability.
Regular maintenance practices, such as thorough cleaning of the cover and inspection of seal integrity, can significantly enhance the lifespan and efficiency of AA systems. According to a report by the National Institute of Standards and Technology, a consistent maintenance schedule can improve the accuracy of measurements by up to 15%. It is essential to monitor exhibition conditions and replace any worn-out parts promptly. Additionally, calibrating the AA instruments following manufacturer recommendations and maintaining a log of all maintenance activities can aid in identifying potential issues before they escalate, ultimately fostering a more productive lab environment. Prioritizing these practices not only ensures optimal performance but also maximizes the return on investment in laboratory equipment.
When working in a laboratory setting, employing the best atomic absorption cover options is crucial for ensuring safety and maintaining the integrity of experiments. Laboratory personnel should familiarize themselves with the safety protocols associated with using covers, which are designed to minimize contamination and exposure to hazardous substances. Utilizing covers that are non-reactive and easy to clean can significantly enhance safety, as they reduce the risk of chemical spillage and protect sensitive equipment from particulate matter.
In addition to selecting appropriate covers, implementing best practices for their use is vital. It is essential to ensure that covers fit securely to prevent any accidental exposure and to regularly inspect them for wear and tear. Training staff on proper usage procedures, such as not removing covers unnecessarily and ensuring that they are placed back immediately after use, can help maintain a safer laboratory environment. Moreover, labeling covers with relevant safety information can serve as a constant reminder of the precautions that must be taken, thereby reinforcing a culture of safety within the laboratory.
This chart illustrates the effectiveness rating of various types of atomic absorption covers used in laboratory settings. Higher ratings indicate better performance and suitability for laboratory use. The ratings were based on materials commonly used in laboratories and their effectiveness in providing safety and operational efficacy.
